We’re all trying to stretch the dollar and get the most out of our money. But can you do that when it comes to cosmetic treatments? The answer: YES! There are so many options out there, yet which do you choose? I’ve compiled my list of the Top 5 Cosmetic Bangs for the Buck. Here […]
-
Posted on: Feb 7 2022
-
By: editor
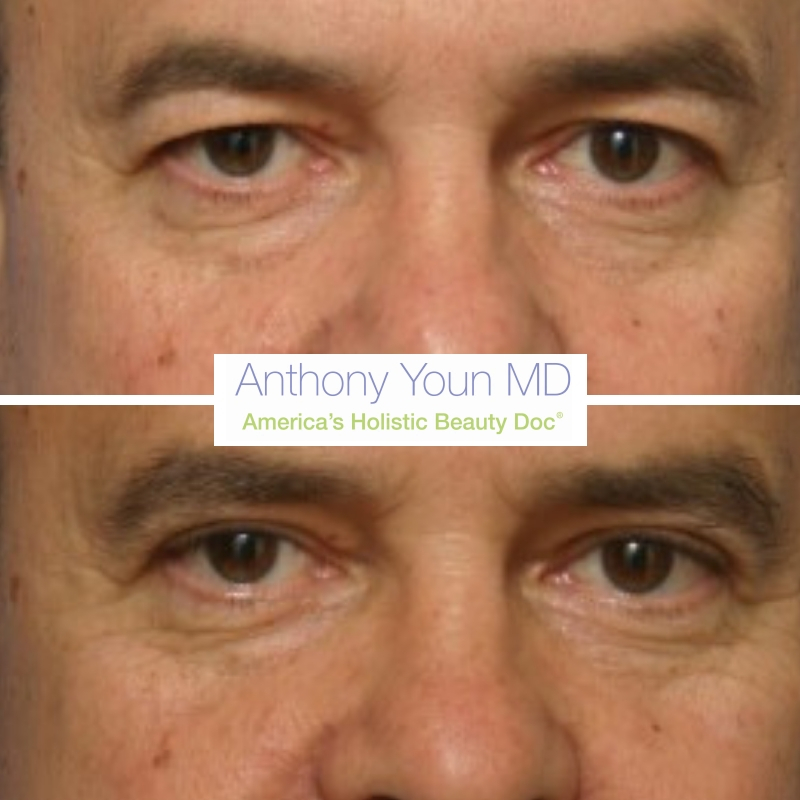
Your eyes are the windows to your soul. They’re also the part of the face which most people complain about to me! Droopy skin, puffy lids, crow’s feet, and wrinkles – our eyes age in so many different ways. Here are some recommendations on how to treat them: Droopy skin of the upper eyelids – […]
-
Posted on: Nov 19 2021
-
By: editor
Treating scars and stretch marks to reduce their appearance isn’t easy, but it’s not impossible! From topical solutions to in-office treatments, I have some recommendations for you to look into. For Scars: Silagen – This is a silicone-based cream that I encourage all of my surgery patients to use. Its works best on newer scars to […]
-
Posted on: Jan 14 2021
-
By: editor
Botox injections are the most popular cosmetic treatments in the world. While I’m sure you know that Botox is great for smoothing wrinkles, you might not know that it can do a WHOLE lot more. Here are some other fascinating things Botox can do: 1. Narrow a wide jaw – This treatment originated in Asia and is […]
-
Posted on: Jun 6 2019
-
By: editor
It’s a question I get asked every day, “What is the biggest bang for my cosmetic buck?” There are so many options out there, which do you choose? I’ve compiled my list of the Top 5 Cosmetic Bangs for the Buck. Here they are: 5. Intense Pulsed Light (IPL) – Do you have sun and age spots […]
-
Posted on: Jan 17 2019
-
By: editor
Is the third time the charm? This month the FDA approved Xeomin® (incobotulinumtoxinA) for the treatment of moderate to severe wrinkles in the glabellar region (frown lines). This is the third botulinum toxin to be approved by the FDA for cosmetic use (following Botox and Dysport). Merz Aesthetics plans to release Xeomin for use in the Spring […]
-
Posted on: Jul 28 2011
-
By: editor
The first real Botox competitor, Dysport, is finally reaching plastic surgeons’ offices here in the U.S. We received our first shipment this week and I’m excited to bring this product to my patients. Like Botox, it’s a Botulinum Toxin Type A and is FDA-approved to temporarily reducing the wrinkles in the glabella (frown lines). Doctors […]
BIG news in the plastic surgery field. The FDA approved the drug Dysport (otherwise known as Reloxin) today for the treatment of fine lines in the glabella (between the brows). This is the first bona-fide competitor to Botox, as it is also a Botulinum Toxin Type A. I’ve mentioned Reloxin in this blog before, as […]